生物相似劑的分子比主要由小分子組成的仿製藥大約1,000倍,而且結構上亦更為複雜。1
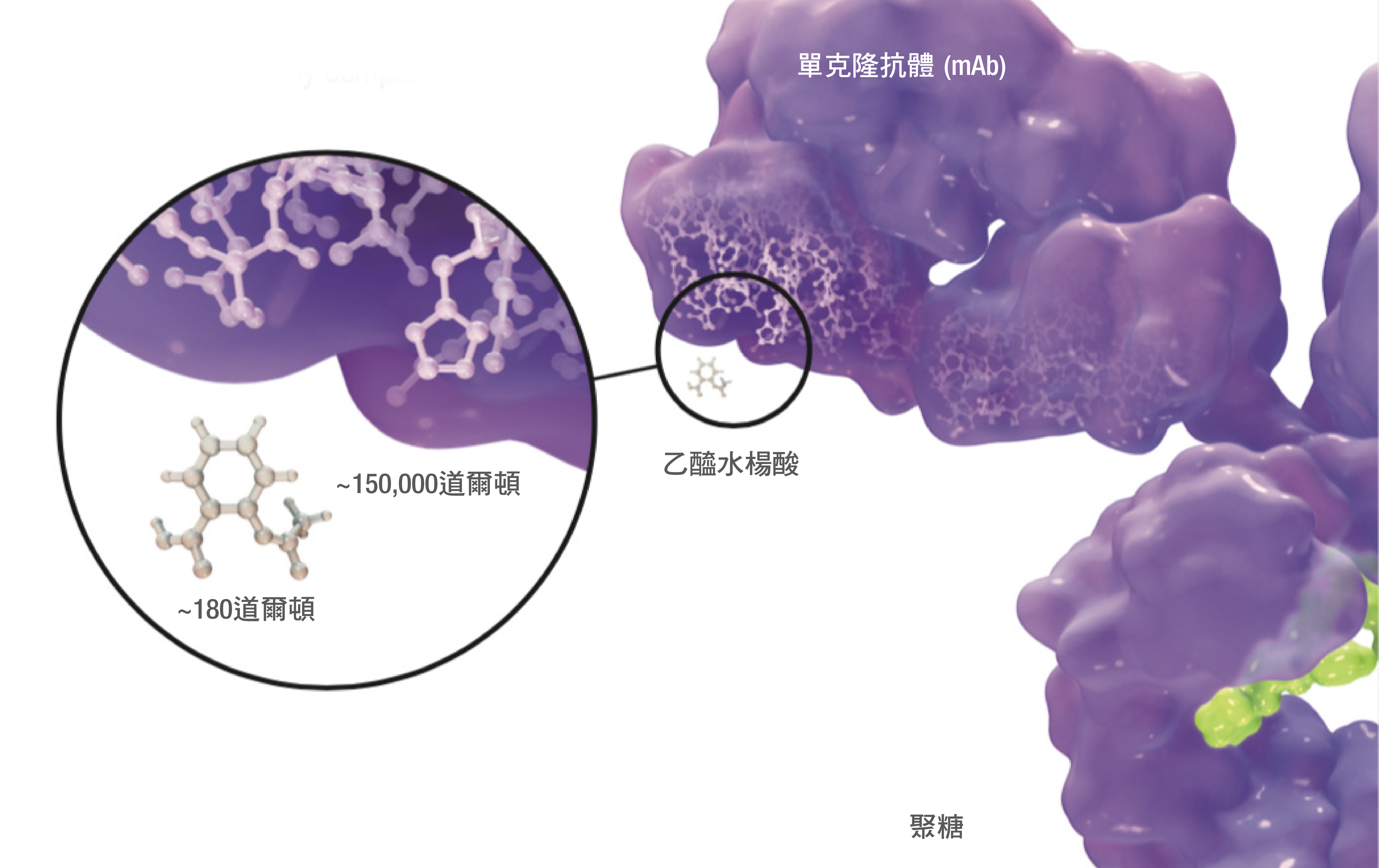
此外,生物相似劑是透過活細胞基因工程所製造,經由自訂生產流程,自行研發新的細胞。因此,生物相似劑不像仿製藥般跟參考藥物完全一模一樣。
因此,開發生物相似劑的過程比仿製藥更加複雜,其監管途徑亦更為嚴格,亦需要臨床數據證實與原廠生物製劑在功效和安全性上沒有臨床意義上的差異。2,3
References: 1. Lee JF, Litten JB, Grampp G. Comparability and biosimilarity: considerations for the healthcare provider. Curr Med Res Opin. 2012;28:1053-1058. 2. Camacho LH, Frost CP, Abella E, et al. Biosimilars 101: considerations for U.S. oncologists in clinical practice. Cancer Med. 2014;3:889-899. 3. Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419. 4. US Food and Drug Administration. Guidance for industry: quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291134.pdf. Accessed October 29, 2019. 5. US Federal Trade Commission. Emerging health care issues: follow-on biologic drug competition: a federal trade commission report. www.ftc.gov/reports/emerging-health-care-issues-follow-biologic-drug-competition-federal-trade-commission-report. Accessed October 29, 2019. 6. EuropaBio. Guide to Biological Medicines: A Focus on Biosimilar Medicines. 2011.