
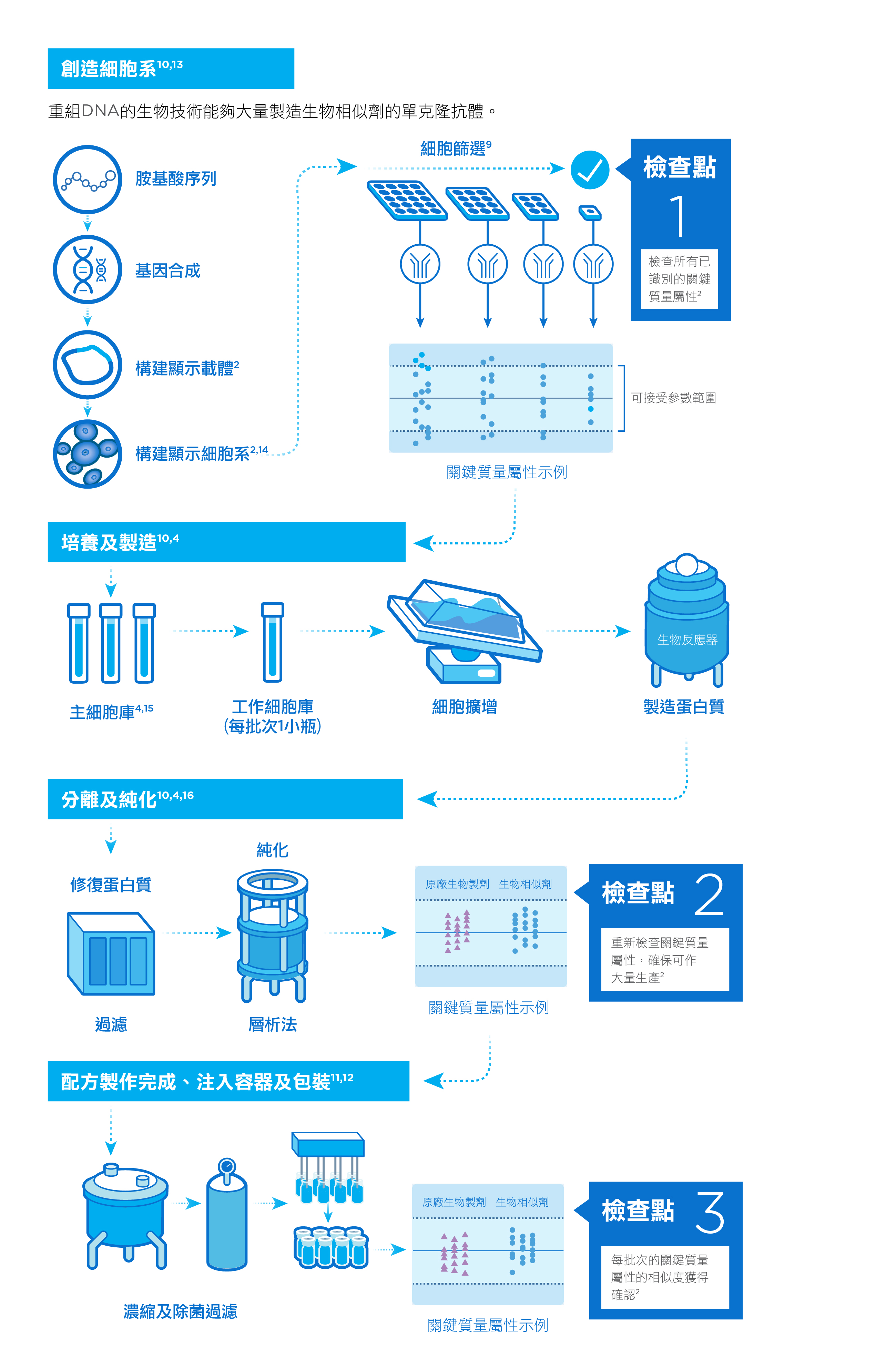
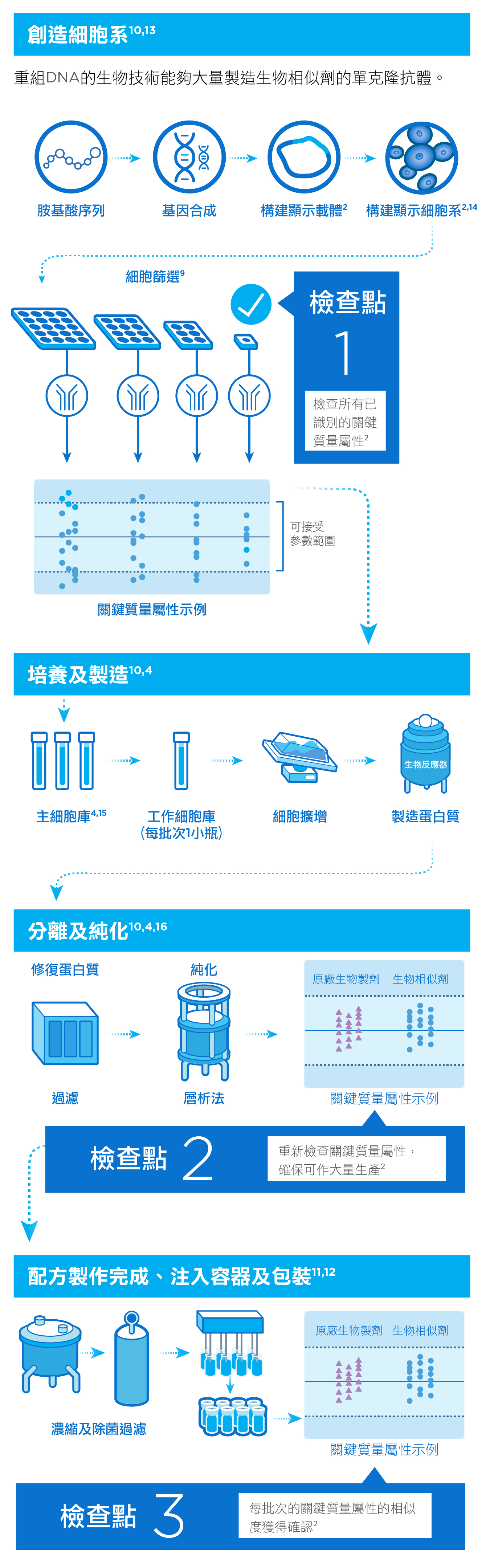
當發展出用作開發生物相似劑的細胞後,製造商會仔細分析細胞所生產出來的分子,並將其關鍵質量屬性與參考生物製劑進行特性比較。關鍵質量屬性對藥物的安全性、藥效、藥物動力學和整體品質有著重要影響 。1,2
生物製劑難以大量生產,因為在製造過程中,環境因素非常重要,溫度和酸鹼值等的輕微變化都足以令藥物變質 。3,4 因此,製造商必須嚴謹監控產品開發的每個步驟,確保藥物的純度、穩定性和藥效達標。只有藥物品質獲得認證,方可進行包裝對外供應。 5
製藥過程中出現的問題或阻礙都可能延誤藥物供應。因此,製造商須與監管機構緊密聯繫,並全面掌握策劃及生產過程,將藥物短缺和供應中斷的風險減到最低。3
“安進可維持優質生物製劑的穩定供應,全賴對患者堅定的承諾、專業知識、先進的設施,並時刻投入資源優化工序。 ”
研製生物相似劑需利用活細胞,並經過多重複雜精細的步驟。然而,生產參考藥物的細胞種類及製造程序由原製藥公司專利擁有,其他製藥公司只能夠透過多次活性對照試驗及實驗,對參考藥物作出比較,從而評估其抗體的生物相似性。透過採購並分析不同批次的參考藥物,有助為日後的結構及功能生物相似性測試建立評估基準。4,6-8

References: 1. US Food and Drug Administration. Guidance for industry: Q8(R2) pharmaceutical development. www.fda.gov/media/71535/download. Accessed May 8, 2020. 2. US Food and Drug Administration. Guidance for industry: quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291134.pdf. Accessed May 8, 2020. 3. Grampp G, Ramanan S. Managing unexpected events in the manufacturing of biologic medicines. BioDrugs. 2013;27:305-316. 4. Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419. 5. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478. 6. Blauvelt A, Cohen AD, Puig L, Vender R, van der Walt J, Wu JJ. Biosimilars for psoriasis: preclinical analytical assessment to determine similarity. Br J Dermatol. 2016;174:282-286. 7. US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed October 29, 2019. 8. McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91:405-417. 9. Kozlowski S. US FDA perspectives on biosimilar biological products. Presented at: 2014 Biotechnology Technology Summit; June 13, 2014; Rockville, MD. www.ibbr.umd.edu/sites/default/files/public_page/Kozlowski%20-%20Biomanufacturing%20Summit.pdf. Accessed May 8, 2020. 10. Desanvicente-Celis Z, Gomez-Lopez A, Anaya JM. Similar biotherapeutic products: overview and reflections. Immunotherapy. 2012;4:1841-1857. 11. Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs. 2014;28:363-372. 12. Bee JS, Randolph TW, Carpenter JF, Bishop SM, Dimitrova MN. Effects of surfaces and leachables on the stability of biopharmaceuticals. J Pharma Sci. 2011;100:4158-4170. 13. Dranitsaris G, Amir E, Dorward K. Biosimilars of biological drug therapies. Drugs. 2011;71:1527-1536. 14. Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. mAbs. 2010;2:480-499. 15. Roger SD. Biosimilars: how similar or dissimilar are they? Nephrology. 2006;11:341-346. 16. Hesse F, Wagner R. Developments and improvements in the manufacturing of human therapeutics with mammalian cell cultures. Trends Biotechnol. 2000;18:173-180.