Biosimilars are up to 1,000 times the size of small-molecule generic drugs, and are far more structurally complex.1
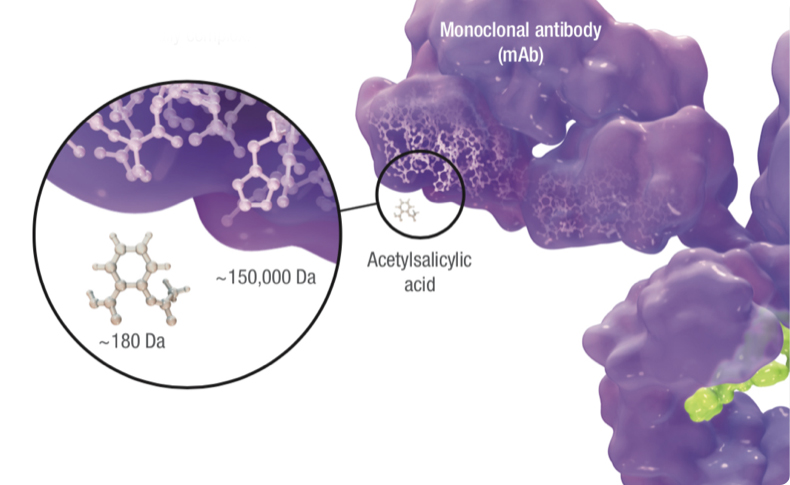
Additionally, biosimilars are manufactured in living cell lines using processes that cannot be exactly replicated from one manufacturer to the next. Therefore, a biosimilar, unlike a small-molecule generic, is not an exact copy of its reference product. The underlying differences in size, complexity, and manufacturing processes are why biosimilars are fundamentally different from generics.
One key reason a biosimilar cannot be identical to its reference biologic is due to post-translational modifications such as glycosylation—the addition of glycans (carbohydrate groups) to a protein within the producing cell. These are unique to each specific cell line and growth conditions and can have a profound impact on the molecule’s biological effects, including drug clearance rates and immunogenicity.2,3
Therefore, biosimilars are more complicated to develop and the regulatory pathway for approval is more complex than for generic drugs.2,3
Differences between generics and biosimilars:
References: 1. Lee JF, Litten JB, Grampp G. Comparability and biosimilarity: considerations for the healthcare provider. Curr Med Res Opin. 2012;28:1053-1058. 2. Camacho LH, Frost CP, Abella E, et al. Biosimilars 101: considerations for U.S. oncologists in clinical practice. Cancer Med. 2014;3:889-899. 3. Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419. 4. US Food and Drug Administration. Guidance for industry: quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291134.pdf. Accessed October 29, 2019. 5. US Federal Trade Commission. Emerging health care issues: follow-on biologic drug competition: a federal trade commission report. www.ftc.gov/reports/emerging-health-care-issues-follow-biologic-drug-competition-federal-trade-commission-report. Accessed October 29, 2019. 6. EuropaBio. Guide to Biological Medicines: A Focus on Biosimilar Medicines. 2011.