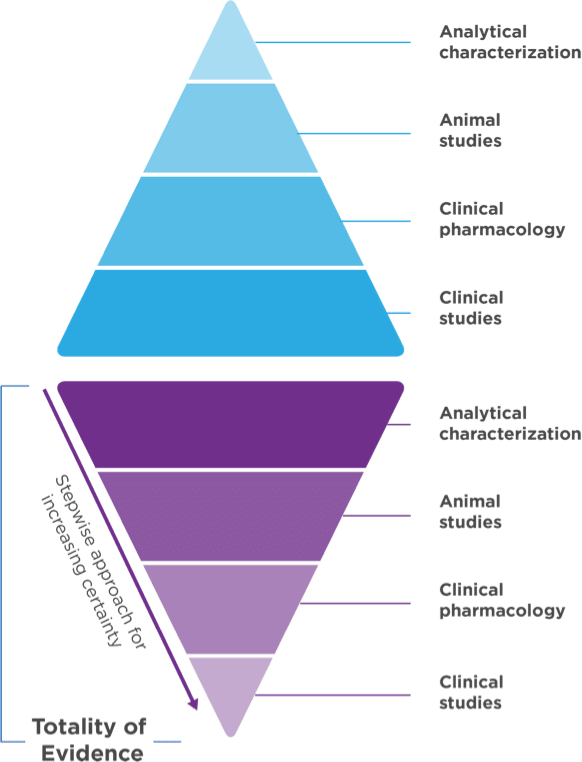
Biosimilar development1,3 Demonstrate no clinically meaningful differences in safety and effectiveness in one or more appropriate conditions of use for which the reference product is licensed. Biosimilar development1,3 Demonstrate no clinically meaningful differences in safety and effectiveness in one or more appropriate conditions of use for which the reference product is licensed.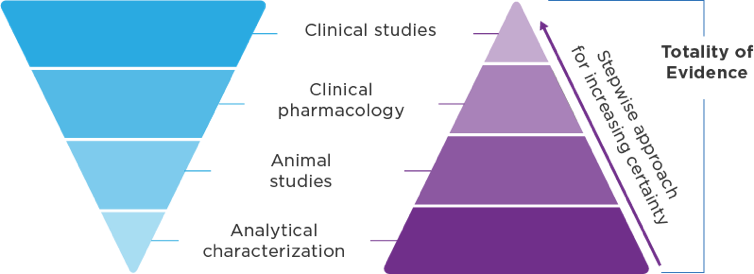
References: 1. US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed October 29, 2019. 2. Conner J, Wuchterl D, Lopez M, et al. The biomanufacturing of biotechnology products. In: Shimasaki C, ed. Biotechnology Entrepreneurship: Starting, Managing, and Leading Biotech Companies. Waltham, MA: Academic Press; 2014:351-385. 3. Blauvelt A, Puig L, Chimenti S, et al. Biosimilars for psoriasis: clinical studies to determine similarity. Br J Dermatol. 2017;177:23-33.