
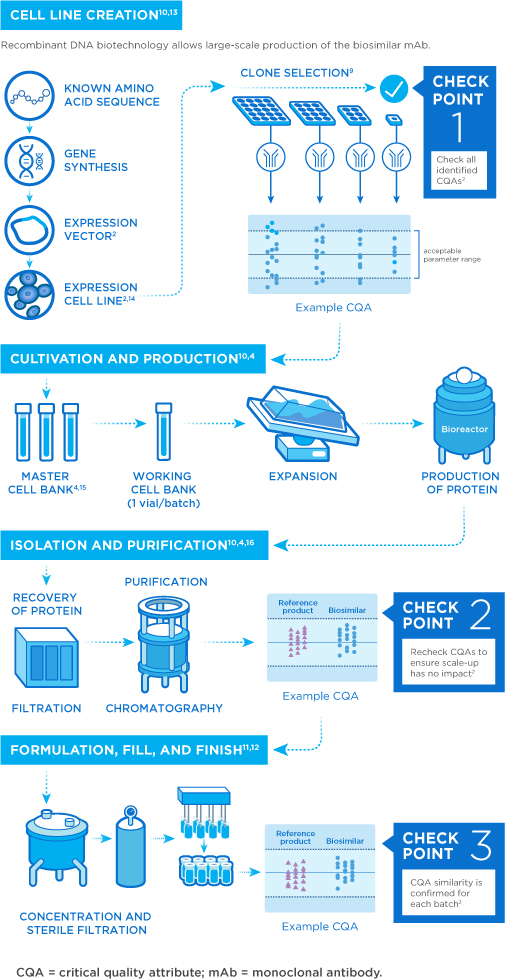
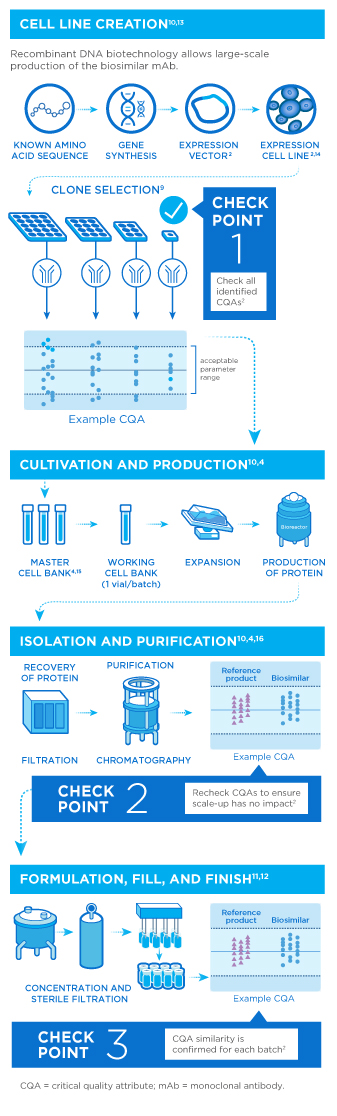
Once a cell line is developed for the biosimilar, the candidate molecule is analyzed and carefully compared to the reference drug using a number of characteristics called critical quality attributes (CQAs). CQAs are features associated with the reference drug that can impact safety, potency, pharmacokinetics, and overall quality.1,2
Biologic medicines are highly sensitive to factors like temperature and pH, making them more difficult to produce on a large scale. Even minor alterations in these conditions may lead to unwanted or unintended changes in the final product.3,4 As such, manufacturers are responsible for monitoring all steps of product development to ensure that the medicine is pure, stable, and has the desired strength. Once its quality has been verified, the medicine is packaged for use.5
Problems or interruptions to the manufacturing process of biologic medicines may not only affect quality and safety, but could also lead to delayed supplies and distribution of urgently needed medicines. As such, along with regulators, manufacturers have a responsibility to ensure strategies are in place to minimize incidences of drug shortages and supply disruption.3
“A reliable supply of quality biologic medicines requires commitment, and highly specialized biologics knowledge and expertise, infrastructure, and capital investment.”
Biosimilars are produced through an intricate, multistep process using living cells. However, the cell line and manufacturing process of the reference product are proprietary to the original manufacturer. Biosimilarity for antibodies can only be established through evaluation of the biosimilar in active comparator clinical trials and experiments with the reference drug. Procurement and analysis of multiple batches of the reference drug establish a baseline to inform subsequent structural and functional biosimilarity tests.4,6-8

References: 1. US Food and Drug Administration. Guidance for industry: Q8(R2) pharmaceutical development. www.fda.gov/media/71535/download. Accessed May 8, 2020. 2. US Food and Drug Administration. Guidance for industry: quality considerations in demonstrating biosimilarity of a therapeutic protein product to a reference product. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm291134.pdf. Accessed May 8, 2020. 3. Grampp G, Ramanan S. Managing unexpected events in the manufacturing of biologic medicines. BioDrugs. 2013;27:305-316. 4. Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411-419. 5. Blackstone EA, Joseph PF. The economics of biosimilars. Am Health Drug Benefits. 2013;6:469-478. 6. Blauvelt A, Cohen AD, Puig L, Vender R, van der Walt J, Wu JJ. Biosimilars for psoriasis: preclinical analytical assessment to determine similarity. Br J Dermatol. 2016;174:282-286. 7. US Food and Drug Administration. Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product. www.fda.gov/downloads/drugs/guidances/ucm291128.pdf. Accessed October 29, 2019. 8. McCamish M, Woollett G. The state of the art in the development of biosimilars. Clin Pharmacol Ther. 2012;91:405-417. 9. Kozlowski S. US FDA perspectives on biosimilar biological products. Presented at: 2014 Biotechnology Technology Summit; June 13, 2014; Rockville, MD. www.ibbr.umd.edu/sites/default/files/public_page/Kozlowski%20-%20Biomanufacturing%20Summit.pdf. Accessed May 8, 2020. 10. Desanvicente-Celis Z, Gomez-Lopez A, Anaya JM. Similar biotherapeutic products: overview and reflections. Immunotherapy. 2012;4:1841-1857. 11. Ramanan S, Grampp G. Drift, evolution, and divergence in biologics and biosimilars manufacturing. BioDrugs. 2014;28:363-372. 12. Bee JS, Randolph TW, Carpenter JF, Bishop SM, Dimitrova MN. Effects of surfaces and leachables on the stability of biopharmaceuticals. J Pharma Sci. 2011;100:4158-4170. 13. Dranitsaris G, Amir E, Dorward K. Biosimilars of biological drug therapies. Drugs. 2011;71:1527-1536. 14. Liu HF, Ma J, Winter C, Bayer R. Recovery and purification process development for monoclonal antibody production. mAbs. 2010;2:480-499. 15. Roger SD. Biosimilars: how similar or dissimilar are they? Nephrology. 2006;11:341-346. 16. Hesse F, Wagner R. Developments and improvements in the manufacturing of human therapeutics with mammalian cell cultures. Trends Biotechnol. 2000;18:173-180.