These therapies have been used to treat millions of patients around the world, and there’s an opportunity to help treat millions more with Amgen’s growing portfolio of biosimilars, both approved and in the pipeline.One of the first companies to develop:2,3
Amgen was one of the first large-scale biotechnology manufacturers, and we’re now one of the largest independent biotechnology companies. We have developed some of the most widely used, quality biologic medicines, with a portfolio totaling more than a dozen medicines across six therapeutic areas: oncology and hematology, cardiovascular disease, nephrology, inflammation, bone health, and neuroscience.
Each step of the way, we’ve refined our biotechnological processes and expertise. Our experience developing and refining our systems reassures the healthcare community that we can provide a reliable supply of our biologic medicines worldwide.4
More recently, we have used human genetic validation whenever possible in the discovery and development process:
Amgen's “biology first” approach, coupled with an array of drug modalities, carries several benefits:

Enhanced likelihood of success

Efficient development of new medicines
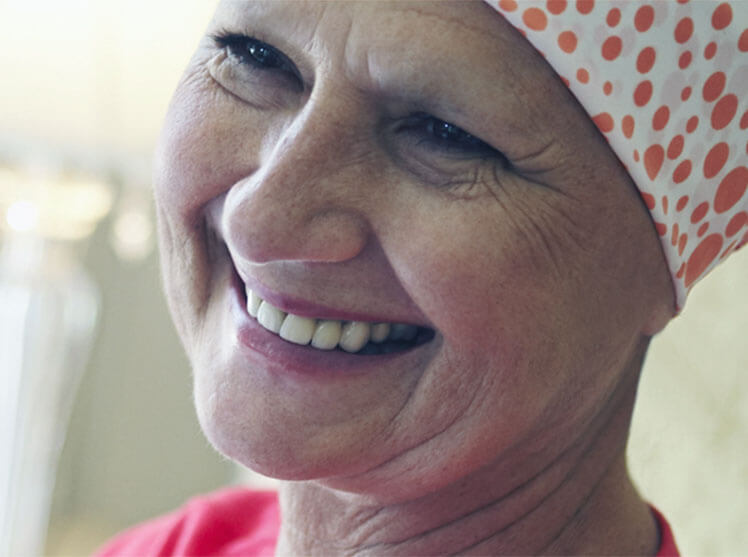
Increased patient options
to new medicines
Many things at Amgen remain unchanged from those early years. We still operate with the same drive, determination, and innovative spirit and values—finding ways to transform new ideas and discoveries, to make life better for millions of patients.

One of our most exciting pursuits is the exploration of biosimilar medicines, which will offer new treatment options for physicians and patients. We are taking our rich biologics experience and putting everything we’ve learned into making quality biosimilar medicines. Not only that, we’re backing it all with our established reputation for quality, reliability, and support services.
References: 1. National Center for Biotechnology Information. EPO Erythropoietin [homo sapiens (human)]. www.ncbi.nlm.nih.gov/gene/2056. Accessed October 29, 2019. 2. Lybecker KM. The biologics revolution in the production of drugs. Fraser Institute. www.fraserinstitute.org/sites/default/files/biologics-revolution-in-the-production-of-drugs.pdf. Accessed October 29, 2019. 3. Nowicki M. Basic facts about biosimilars. Kidney Blood Press Res. 2007;30:267-272. 4. Schipper R, et al. Assessment of drug supply reliability in Europe by evaluating on time in full (OTIF) delivery. J Oncol Pharm Pract 2018;248(Suppl):32-33. Abstract 32.